Saffron And Its Constituents For Pain Management
Pain can become a chronic and deliberating experience with a significant burden. In preclinical and clinical studies, Saffron (Crocus sativus L.) has shown analgesic properties. Considering the unsatisfactory results of current therapeutic management for chronic pain conditions, we aimed to review Saffron's analgesic activity and underlying mechanisms. Saffron showed ant-nociceptive activities in formalin, carrageenan, and capsaicin-induced experimental pain models. Saffron analgesic activities affected several targets, including ion channels of nociceptors; the adrenergic system and central histaminic system; inhibition of inflammatory pathways, apoptotic pathways, and oxidative stress; regulation of NO path, and the endocannabinoid system. Clinical studies showed analgesia of Saffron in rheumatoid arthritis, after-pain following childbirth, dysmenorrhea, and fibromyalgia. Our literature review showed that Saffron can be beneficial as an adjunct therapy to commonly used analgesics in practice, particularly in chronic pain conditions.
This is a preprint edition of Ali Mohammad Pourbagher-Shahri and Fatemeh Forouzanfar's paper.
1 | INTRODUCTION
Since time immemorial, herbal plants have been the primary source of medications for humans. Herbal medicines have developed biological equilibrium by containing natural active materials and other substances. The absence or low risk of side effects has brought much attention to herbal remedies in recent decades of research in modern medicine (Setty & Sigal, 2005; Y. Wang et al., 2021). A great example of these herbs is saffron, obtained from the flower stigmas of Crocus sativus L., which belongs to the Iridaceae family. The original Persian name for saffron is "Za'afarn," which means golden flowers. The high price of saffron has made saffron called "Red Gold" in the producer countries (Cid-Pérez et al., 2021; José Bagur et al., 2017).
2 | CHEMICAL COMPOSITIONS OF SAFFRON
Saffron composition includes moisture (10%), protein (12%), fat (5%), minerals (5%), crude fiber (5%), and carbohydrates (63%) (Shahi et al., 2016). The two main parts of the C. sativus L. plant are the tepal and stigma. Stigma has over 150 volatile and non-volatile compounds, including lipophilic and hydrophilic carbohydrates, proteins, amino acids, minerals, mucilage, starch, gums, vitamins, pigments, alkaloids, saponins, safranal, Picrocrocin, and several other compounds (Shahi et al., 2016; Winterhalter & Straubinger, 2000). Among these compounds, the ones that are mainly responsible for bioactivities of interest for human health are crocin, crocetin, Picrocrocin, safranal, and flavonoids, including quercetin and kaempferol (Shahi et al., 2016).
3 | BRIEF HISTORY AND PHARMACOLOGICAL EFFECTS OF SAFFRON
Planting of C. sativus L. has been a joint endeavor among farmers. Saffron, a perennial spicy herb, is commonly used to provide color, flavor, and aroma to foods and drinks (Khorasanchi et al., 2018). Also, in ancient times, saffron was used to color clothes expensively and create odors for perfumes. In folk medicine, saffron was used as an aphrodisiac agent, expectorant, laxative, antispasmodic, antidepressant, sedative, and dysmenorrhea. In the food industry, saffron is used as an odor, tasting, and coloring agent in different foods on industrial scales (Shahi et al., 2016). Growing evidence from pharmacological studies has displayed that saffron or its ingredients have health-promoting properties effects including: neuroprotective (Maggi et al., 2020), anxiolytic (Pitsikas, 2016), antidepressive (Musazadeh et al., 2022), antidiabetic (Yaribeygi et al., 2019), antioxidant (Mashmoul et al., 2013), antiinflammatory (Fernández-Albarral et al., 2019), analgesic (Ait Tastift et al., 2022), anti-hypertensive (Kadoglou et al., 2021), hypolipidemic (Asdaq & Inamdar, 2010), protection against ischemic stroke (Azami et al., 2021), and anti-tumor (Patel et al., 2017).
4 | PAIN
The ability to detect dangerous stimuli through pain is essential to our biological body system and necessary for our well-being and survival (Raffaeli & Arnaudo, 2017). However, the pain mechanisms are sometimes altered to lead to hypersensitivity, and once a useful phenomenon becomes a chronic and deliberating experience. A significant and growing global burden of pain exists, as for every five patients, one experiences pain. Also, chronic pain is diagnosed in 1 of 10 patients every year. Expectedly, pain is the most common reason for seeking medical care (Fishman, 2007; Goldberg & McGee, 2011). Neuropathic pain is a complex, chronic, heterogeneous pain state arising from a lesion/disease affecting the somatosensory system (Finnerup et al., 2021; Forouzanfar et al., 2019). Several fundamental biological events at multiple levels of the nervous system, from the peripheral nervous system (PNS) to the central nervous system (CNS), are involved in the complex processes of pain transduction and perception (Argoff, 2011; Asgharzade et al., 2020; Forouzanfar, 2022). Two terms exist in the experience of pain in living beings: pain itself and nociception. Pain is a CNS event, while nociception is a mechanical process in the PNS for reporting tissue damage to the CNS.
An "acute," typical, and ordinary human experience of pain starts within the PNS as a noxious stimulus (stimulations capable of inducing tissue damage) activates a subpopulation of primary sensory neurons with specialized nerve endings called nociceptor (Bingham et al., 2009; Woolf, 2004). Nociceptors exist in different tissues throughout the body, including skin, fascia, muscle, tendon, blood vessels, joints, viscera, meninges, and bone (Basbaum et al., 2009). Nociceptors mainly exist in two types: A-fiber and C-fiber. The former handles the acute, well-localized sharp pain and has myelinated axons and higher conduction speed of action potentials. C-fibers manage the slower, sluggish, throbbing, poor-localized pain because of having unmyelinated axons and slow conduction of action potential (Basbaum et al., 2009; Besson, 1999; Venkatachalam & Montell, 2007). In certain types of pain, under most chronic conditions, the involvement of various physiological pathways from the transmission to perception lowers the success rate of single-agent analgesic therapy. Because chronic pain causes are poorly understood, and patients also suffer from comorbid conditions such as anxiety and depression, chronic pain remains poorly managed (Attal et al., 2011; Courtney et al., 2017; Markenson, 1996; Ossipov et al., 2014). Long-term use of analgesic drugs has its limitations, such as adverse effects and drug-to-drug interactions (Mercer Lindsay et al., 2021; Negah et al., 2021). Medicinal plants are a source of an infinite range of phytochemicals that have consistently been shown to have analgesic therapeutic properties (Forouzanfar et al., 2023; Negah et al., 2023; Rakhshandeh et al., 2021). A critical evaluation of the clinical data regarding the adverse effects has shown that herbal remedies are generally better tolerated than synthetic medications (Izzo et al., 2016). So, a therapeutic agent with analgesic potentials added to current pain treatments can significantly benefit. Considering the therapeutic potential of saffron as an analgesic, its usage in preclinical and clinical studies is reviewed here.
5 | SEARCH STRATEGY
A literature search was performed using the following databases and search engines: PubMed, Scopus, Science Direct, Embase, EBSCO, Cochrane Library, and Google Scholar. The keyword search strategy employed was ["crocus sativus L." OR "saffron" OR "crocin" OR "safranal" OR "crocetin" OR "picrocrocin"] AND ["pain" OR "nociception" OR "analgesic" OR "analgesia" OR "hyperalgesia" OR "allodynia"]. Full texts of identified articles were obtained, and relevant bibliographies were manually searched to identify additional articles.
6 | ANTINOCICEPTIVE AND ANALGESIC ACTIVITIES OF SAFFRON'S EXTRACTS ACCORDING TO VIVO STUDIES
The antinociceptive and antiinflammatory effects of aqueous and ethanolic extracts of C. sativus L. stigma and petals in mice were examined. The study used various tests, such as the hot plate test, writhing test, xylene-induced ear edema, and formalin-induced inflammation, to evaluate the effects of these extracts. The results showed that stigma and petal extracts have antinociceptive effects in chemical pain tests. However, in the hot plate test, C. sativus extracts had no significant central-acting antinociceptive activity. The stigma extracts showed activity against acute and chronic inflammation, while the petal ethanolic extract showed antiinflammatory activity in the regular inflammatory test (Hosseinzadeh & Younesi, 2002). One research revealed increased spinal cord tumor necrosis factor α (TNFα) and interleukin-1β (IL-1β) levels in rats' post-chronic constriction injury (CCI), indicative of neuropathy and oxidative stress. These levels were notably reduced with C. sativus extract treatment. This treatment decreased malondialdehyde (MDA) levels while restoring glutathione (GSH) content, indicating the plant's potential role in moderating oxidative stress, inflammatory cytokines, and mitochondrial apoptosis pathways. Hence, the study associates saffron's antinociceptive effects with these biological adjustments in CCI rats (Amin et al., 2014). Depression often accompanies neuropathic pain, impacting the quality of life significantly (Nicholson & Verma, 2004).
Antidepressants can help to mitigate pain independently of their depression-targeted effects (Matsuzawa-Yanagida et al., 2008). One study focused on the potential benefit of combining saffron extract with amitriptyline, an antidepressant, to ease depressive and pain symptoms in CCI rats. They administered varying amitriptyline and saffron extracts to rats for seven days, observing significant improvement in response to pain stimuli with amitriptyline doses of 10 and 30 mg/kg. A 100 mg/kg of saffron extract temporarily relieved pain on certain days. Combining low-dose amitriptyline with saffron extract amplified the effect (Amin et al., 2017). One study explored the analgesic properties of C. sativus stigma extract, derived from saffron, in rodent models. Three tests (hot plate, writhing, and formalin tests) were used to assess the antinociceptive effects of C. sativus stigma extract. Oral administration of C. sativus stigma extract, up to 2000 mg/kg, did not lead to any mortality or changes in animal behavior, blood parameters, or liver and kidney histological structures. C. sativus stigma extract showed a dose-dependent antinociceptive effect to thermal stimuli (central effect) and a peripheral analgesic effect in response to acetic acid-induced contortions. The formalin test confirmed that the C. sativus stigma extract analgesic effect worked centrally and peripherally. The analgesic activity of C. sativus stigma extract could be reversed entirely or partially by administering receptor antagonists such as naloxone, atropine, haloperidol, yohimbine, and glibenclamide. This suggests that C. sativus stigma extract pain-relieving effect is influenced by a variety of physiological systems, including opioidergic, adrenergic, and muscarinic systems at both peripheral and central levels, the dopaminergic system, and the regulation of ATP-sensitive K+ channels at the spinal level. The study concludes that C. sativus stigma extract's analgesic mechanism involves both an antiinflammatory effect and a modulation of the electrical signals of nociceptors through several physiological pathways (Ait Tastift et al., 2022). These studies have shown that saffron extracts can produce antinociceptive and analgesic effects in various pain models. It should be noted that there are some differences between aqueous and ethanolic extracts from a plant or any other material. A study compared phytochemicals and antioxidants in various saffron extracts. The 80% ethanolic extract extracted for 24 had the most phenolic compounds. All extracts could neutralize a specific free radical, but the ethanolic extract was the most effective. Antioxidant capacity and phytochemical content were influenced by solvent type and extraction time. There was a strong positive correlation between antioxidant activity and phenolic and flavonoid contents. Chromatography showed varying amounts of crocins, Picrocrocin, and safranal across different solvents (Rahaiee et al., 2015). So, based on which extract is used in every study, the effects on pain responses could be other.
7 | ANTINOCICEPTIVE AND ANALGESIC ACTIVITIES OF CROCIN ACCORDING TO VIVO STUDIES
Crocins are glucosylated apocarotenoids found in the stigmas of C. sativus and other Crocus species, contributing to their color variations. They have also been discovered in the tepals (parts of the flower that are neither clearly petal nor sepal) of yellow Crocus species. β-carotene is identified as the precursor of these crocins. The presence of crocins in tepals suggests a role in attracting pollinators and identifies tepals as new sources of crocins, which have applications in medicine, cosmetics, and coloring industries. Regarding biosynthesis, phytoene synthase is a critical enzyme in apocarotenoid biosynthesis in these tissues.
The accumulation of crocetin in the stigma begins early in development, peaks in the red-immature stage, and decreases after that (Rubio Moraga et al., 2013). Formalin, capsaicin, and carrageenan are frequently used in pain studies. Formalin, when injected, initiates a two-stage pain response: an immediate, short-lived one due to direct action on nociceptive neurons, followed by a longer, inflammatory phase involving the release of histamine, serotonin, bradykinin, and prostaglandins, causing central sensitization (Hunskaar & Hole, 1987; Raboisson & Dallel, 2004; Tjølsen et al., 1992). Capsaicin, found in chili peppers, triggers acute inflammatory responses. It activates a unique group of sensory neurons, transient receptor potential vanilloid member 1 (TRPV1), eliciting the peripheral release of several inflammatory mediators like calcitonin gene-related peptide (CGRP), neurokinin A/B, somatostatin, vasoactive intestinal polypeptide (VIP), and substance-P, which induce local inflammation through the release of pro-inflammatory cytokines (Ilie et al., 2019; Muley et al., 2016). Carrageenan, a polysaccharide derived from red edible seaweeds, is used in studies on acute peritoneal inflammation. It promotes vasodilation, plasma exudation, and recruitment of leukocytes, mainly neutrophils. Like formalin, carrageenan produces a biphasic pattern (Phase I: up to 1–2 h, phase II: up to 2–6 h) of paw edema in rats (Posadas et al., 2004). The opioid system plays a pivotal role in pain mechanisms. It modulates pain perception, as evidenced by its influence on animal models' measures, such as paw lick and jump latencies. One study showed that the antinociceptive effects (pain-reducing effects) of DAMGO (a μ-opioid receptor agonist) and RB101 (a mixed inhibitor) were potentiated by BDNL, a cholecystokinin (CCK) agonist. This suggests an interaction between the opioid system and the CCK system in regulating thermal nociception, a type of pain perception. CCK is a peptide with antinociceptive properties. The study found that BDNL, a CCK agonist, increased the antinociceptive effects of DAMGO and RB101, indicating that CCK can modulate the opioid system and pain perception. Research uncovered a regulatory relationship between CCK and opioid systems in pain management. Activation of CCKB receptors decreases, while CCKA receptor activation increases endogenous enkephalins. These dynamics suggest CCK agonists might lower the analgesic opiate dosage, reducing side effects (Schutte et al., 1997). The paper by Tamaddonfard and HamzehGooshchi (2010a) investigated the effects of crocin on different response phases of formalin-induced pain in rats and the potential interaction of crocin with the endogenous opioid analgesic system. The formalin test involved the injection of formalin into a rat's paw and measuring the time spent licking and biting the injected paw as a measure of pain. Morphine significantly suppressed both phases of formalin-induced pain, which was prevented by naloxone, a competitive antagonist of opioid receptors. Crocin was found to attenuate pain in a dose-dependent manner. However, naloxone did not reverse the suppressive effect of crocin on pain. Additionally, crocin at a dose of 100 mg/kg significantly increased the antinociceptive effect of morphine. At a high 400 mg/kg dose, crocin significantly suppressed locomotor activities (Tamaddonfard & HamzehGooshchi, 2010a). Tamaddonfard et al. (2015) studied crocin's effect on orofacial pain in rats induced by capsaicin injections. Measuring time spent on face rubbing/grooming as a pain indicator, they found that injections of crocin, morphine, or a combination reduced the pain response. Naloxone blocked morphine's effect but not crocin's, confirming that crocin's analgesic effect does not involve central opioid receptors. One study (Tamaddonfard et al., 2013) explored crocin, safranal, and diclofenac's impact on carrageenan-induced inflammation in rat paws. Higher doses of crocin and safranal curbed the inflammation and neutrophil infiltration while mitigating cold and mechanical allodynia and hyperalgesia. However, lower doses did not exhibit these effects. Their antiinflammatory and antinociceptive outcomes were comparable to diclofenac. One study (Erfanparast et al., 2015) examined crocin and safranal's impacts on orofacial pain in rats, measured by face rubbing duration. The pain was created by formalin injection in the upper lip. High doses of these compounds influenced phase II of the pain response, but lower doses only had an effect when combined with low amounts of diclofenac or morphine. Opioid tolerance is a complex process involving several mechanisms. One key mechanism is the alteration in the opioid receptor function, which can occur through receptor desensitization or internalization. This is where the receptor's response to the opioid is reduced or removed from the cell surface, decreasing the opioid's effect (Williams et al., 2013). Another mechanism is the upregulation of the cyclic AMP (cAMP) pathway. Opioids inhibit the cAMP pathway, but with chronic opioid use, the body compensates by increasing the activity of this pathway. When the opioid is removed, the increased activity of the cAMP pathway leads to withdrawal symptoms (Nestler, 2004). Neuroplastic shifts, such as altered glutamate neurotransmission and N-methyl-D-aspartate (NMDA) receptor amplification, contribute to opioid tolerance, inducing a hyperexcitable state in the nervous system, which influences tolerance and withdrawal symptoms (Trujillo & Akil, 1991). Morphinetriggered neuronal apoptosis, involving pro-apoptotic protein activation and antiapoptotic protein suppression, may also factor into opioid tolerance (Campbell & Meyer, 2006). One research (Tamaddonfard & Hamzeh-Gooshchi, 2010b) studied the impact of crocin in conjunction with morphine on acute corneal pain in rats. The pain was instigated via a saline solution on the cornea, with pain measured via eye wipe frequency. Both intraperitoneal (IP) and intracerebroventricular crocin injections noticeably reduced eye wipes, showing pain relief. These effects were not reversed by naloxone, hinting that crocin's analgesic mechanism may bypass the endogenous opioid analgesic system. Morphine's antinociceptive impacts were notably enhanced with crocin. Brain-derived neurotrophic Factor (BDNF) influences pain modulation through tropomyosin-related kinase B receptors, intensifying NMDA receptors and enhancing neurotransmitter release, leading to hyperalgesia. BDNF also boosts microglial NMDA receptor activity during neuropathic pain (Chen et al., 2014). Safakhah et al. (2020) studied crocin's effects on morphine tolerance and serum BDNF levels in rats with neuropathic pain. Morphine tolerance emerged post-neuropathy induction. Crocin improved morphine's analgesic impact, reducing mechanical allodynia in affected rats, though its higher dose failed to replicate this. Both morphine and crocin decreased serum BDNF levels. Crocin could potentially preserve morphine's analgesic efficiency and prevent morphine tolerance in neuropathic pain, seemingly not via BDNF. One study (Safakhah et al., 2016) examined saffron and crocin's impacts on neuropathic pain in rats induced by CCI. Post nerve lesion, they began administering these substances. By day 26, saffron and a higher dose of crocin (30 mg/kg) lessened thermal hyperalgesia and mechanical allodynia, persisting until day 40, while a lower crocin dose (15 mg/kg) had no effect.
Calcitonin gene-related peptide (CGRP), a neuropeptide involved in nociceptive pathways, is linked to different pain types and found in elevated levels in subjects with musculoskeletal pain, suggesting its role as a neuromodulator in non-headache pain (Schou et al., 2017). Karami et al. (2013) studied crocin's impact on CGRP levels in rats with chronic pain from spinal cord contusion (SCI). They utilized various behavioral tests to assess the rats' condition. Post-injury crocin treatment curbed the SCI-induced CGRP surge, improving mechanical and locomotor recovery tests and the BBB score, but it did not significantly affect the hot plate test. The study highlights crocin's positive effects on chronic pain via CGRP reduction. The endocannabinoid system, which includes cannabinoid receptors and endogenous ligands, is integral to pain modulation. Endocannabinoids like anandamide and 2-arachidonoylglycerol regulate pain, dampening responses to painful stimuli via cannabinoid CB1 and CB2 receptors. Researchers are studying endocannabinoid modulation via inhibiting hydrolysis and uptake, comparing this with synthetic endocannabinoids in various pain models and examining their potential for pain therapy (Guindon & Hohmann, 2009). The interaction between crocin and the cannabinoid system was investigated in a study by Vafaei et al. (2020) in CCI-induced neuropathic pain in rats. Crocin was administrated both peripherally and centrally (intracerebroventricularly). Crocin reduced neuropathic pain in the rats, and this effect was inhibited when a cannabinoid receptor antagonist (AM 251) was administered before crocin. This suggests that crocin's pain-relieving effects may be partly mediated through the cannabinoid system. The researchers also noted that the pain-relieving effect of crocin was more significant when administered centrally rather than peripherally, which they speculated could be because of the first-pass effect or partial degradation of crocin following peripheral administration (Vafaei et al., 2020). Peripheral neuropathy, a debilitating condition affecting nearly half of all adults with diabetes, leads to considerable morbidity, including pain, foot ulcers, and limb amputation. The American Diabetes Association advocates for proactive screening and management, including glycemic control, foot exams, pain management, and lifestyle changes. Recognizing the significant health burden, more vigilant strategies are crucial (Hicks & Selvin, 2019). Farshid and Tamaddonfard's study (2015) explored the impact of crocin and safranal, alone or combined with insulin, on peripheral neuropathy in diabetic rats. Results showed these treatments ameliorated streptozotocin-induced symptoms, including cold allodynia, edema, sciatic nerve degeneration, and hyperglycemia, with the most notable improvements observed with combined treatments. Sleep deprivation has been shown to exacerbate pain sensitivity, a phenomenon known as hyperalgesia. Studies have found that even modest reductions in sleep time can lead to hyperalgesia the following day (Roehrs et al., 2006). This is thought to occur because of the impairment of descending pain-inhibition pathways that are important in controlling and coping with pain (Choy, 2015). Poor sleep quality has been identified as a risk factor for the development of chronic widespread pain (de Oliveira et al., 2017). These findings suggest a complex interplay between sleep and pain, highlighting the importance of good sleep hygiene in preventing and managing chronic pain conditions (Zhao et al., 2017). Rezaei et al. (2020) evaluated the effects of crocin on pain perception in sleep-deprived rats. A programable sleep deprivation device was used to induce a 72-hour extended obligatory sleep deprivation in rats. The rats were then subjected to a hot plate test to measure their pain perception. The results showed that sleep deprivation significantly decreased the latency to noxious thermal stimulus compared with the control group. However, both morphine and crocin significantly increased latency in the sleep-deprived animals, showing that these substances could attenuate hyperalgesia induced by sleep deprivation. The researchers concluded that crocin could be an analgesic for individuals suffering from secondary insomnia and pain (Rezaei et al., 2020). Joint pain from arthritis significantly impairs mobility and overall quality of life (Forouzanfar et al., 2022). Rheumatoid arthritis, an autoimmune disease causing multi-joint inflammation, has been linked to the Wnt signaling pathway, essential for joint and skeletal development and homeostasis. Dysregulation of this pathway may contribute to arthritis conditions, suggesting potential therapeutic interventions. However, the complexity of the Wnt cascades causes more research because of potential side effects (Lories et al., 2013). One study demonstrated that crocin significantly eased pain in arthritis-induced rats, lowering pain-related factors (Wnt5a and β-catenin, TNF-α, IL-1β) and glial activation. The effects of crocin were hindered by Foxy5, a Wnt5a activator, and hyperalgesia in the rats was reduced by a Wnt5a inhibitor (J.-F. Wang et al., 2020). Osteoarthritis, a complex condition causing progressive cartilage loss and inflammation, is a major global source of pain, disability, and economic burden (Loeser et al., 2012). It is characterized by heightened oxidative stress and inflammation, leading to chronic inflammation and the release of inflammatory mediators, which induce further oxidative stress, causing cartilage degradation and joint malfunction (Ansari et al., 2020). One study (Lei et al., 2017) explored crocin's therapeutic effects on osteoarthritis in rats' post-meniscectomy surgery. Findings showed that crocin significantly mitigated joint pain, reduced muscular interleukin-6 levels, increased citrate synthase activity, and increased myosin heavy chain IIα expression. It curbed muscular lipid peroxidation and nuclear factor-erythroid factor 2-related factor 2 (Nrf2) expression while enhancing glutathione production and activity. This suggested crocin may ease osteoarthritis symptoms by reducing oxidative stress and inflammation.
8 | ANTINOCICEPTIVE AND ANALGESIC ACTIVITIES OF CROCETIN ACCORDING TO VIVO STUDIES
Crocetin is a crucial commercially available constituent of saffron and is produced in biological systems by hydrolysis of crocin as a bioactive metabolite (Guo et al., 2022). One study (Erfanparast et al., 2020) examined crocetin's pain-relief effects on rats with formalin-induced orofacial pain. Intra-fourth ventricle injections of crocetin lessened the pain, while yohimbine (alpha2-adrenergic blocker) increased it, negating crocetin's pain relief. Conversely, famotidine (H2 histaminergic blocker) did not affect pain intensity but hindered crocetin's relief. Findings indicated potential involvement of central H2 histaminergic and alpha-2 adrenergic receptors in crocetin's pain relief.
Neuropathic pain development is partly because of oxidative stress (Guedes et al., 2006). Post-nerve injury, there is an upsurge in nitric oxide synthase (NOS) activity and a drop-in superoxide dismutase (SOD) activity, leading to a higher concentration of damaging peroxynitrite. Studies have shown inconsistent results regarding changes in the levels of two SOD isoforms (CuZnSOD and MnSOD) after nerve injury (Rogério et al., 2005; Rosenfeld et al., 1997; Yu, 2002). The effects of crocetin on SOD levels in a mouse model of neuropathic pain induced by spared nerve injury (SNI) were examined. The researchers discovered that crocetin treatment suppressed pain responses and reduced the expression of pro-inflammatory cytokines. While it did not affect CuZnSOD activity, crocetin significantly restored MnSOD activity in the spinal cord and sciatic nerve. This restoration, along with a reduction in thermal allodynia, suggested that crocetin's pain-relieving effect may be attributed to oxidative stress inhibition (X. Wang et al., 2017).
9 | ANTINOCICEPTIVE AND ANALGESIC ACTIVITIES OF SAFRANAL AND PICROCROCIN ACCORDING TO VIVO STUDIES
Safranal and Picrocrocin are two important compounds found in saffron, contributing to its unique aroma and taste. Safranal is a monoterpene aldehyde characterized by a six-membered ring with a formyl group and a side chain comprising a double bond and an aldehyde group (Caballero-Ortega et al., 2004). It is primarily responsible for the distinctive aroma of saffron. Safranal is produced from the degradation of Picrocrocin during the drying process of saffron stigmas (Maghsoodi et al., 2012). Picrocrocin, on the other hand, is a monoterpene glycoside. It is characterized by a glucose molecule attached to a cyclohexane structure with a double bond and an aldehyde group (Predieri et al., 2021). Picrocrocin is the primary compound responsible for the bitter taste of saffron (Chrysanthou et al., 2016). Both safranal and Picrocrocin are sensitive to heat, light, and oxygen, and their levels can significantly influence the quality of saffron (Hosseini et al., 2018; Maqbool et al., 2022). The antinociceptive properties of safranal were investigated. The study used hot-plate, writhing, and formalin tests in mice to determine the antinociceptive activity. The results show that safranal at certain doses inhibits the abdominal constrictions induced by acetic acid (writhing test) and increases the pain threshold of mice at the hot plate test when performed 30 min after formalin injection to the hind paw. They reported that safranal decreased pain-related behaviors in phase I at higher doses, and lower doses decreased phase II of the formalin test (Hosseinzadeh & Shariaty, 2007). TRP channels, a family of versatile excitatory ion channels, play a pivotal role in perceiving harmful chemical, mechanical, and thermal stimuli, with specific channels highly prevalent in certain sensory nociceptive neurons (Kobayashi et al., 2005; Nassini et al., 2014). Two prominent ones are TRPA1, associated with TRPV1 and TRPV4, and responsible for releasing proinflammatory neuropeptides CGRP and substance P. These neuropeptides mediate neurogenic inflammation, with CGRP crucial in migraine pain (Kobayashi et al., 2005; Nassini et al., 2014; Nilius & Szallasi, 2014). Activation of TRPA1 is thought to be involved in phase II of the formalin test (McNamara et al., 2007). Li Puma et al.'s research (2019) focused on safranal and Picrocrocin's impact on TRPA1 channels in various species. Findings revealed that safranal and Picrocrocin interact with these channels, showing greater potency. However, another saffron constituent, crocin, was inactive. Safranal and Picrocrocin activated TRPA1, leading to calcium responses and currents in human cells and rat and mouse dorsal root ganglion neurons. Genetic deletion or pharmacological inhibition of TRPA1 curtailed safranal-induced CGRP release and acute nociception in mice, but this did not affect Picrocrocin. Research suggested that safranal might function as a partial TRPA1 agonist, given that safranal-induced calcium response was less than that of AITC, a TRPA1 activator. Additionally, safranal mitigated the contractile effects in rat urinary bladder isolated strips after exposure to high capsaicin concentrations and a combination of NK1 and NK2 receptor antagonists. When administered for a week, Saffron's extract and safranal reduced mechanical allodynia, thermal hyperalgesia, and thermal allodynia in a dose-dependent manner in CCI rats. To summarize, safranal has shown potential in reducing nociception by selectively desensitizing TRPA1 channels and reducing neuronal excitation caused by CGRP release (Li Puma et al., 2019). Considering these potential therapeutic applications of safranal, given its ability to attenuate TRPA1-mediated responses, safranal could be used for the development of new treatments for conditions associated with TRPA1 channels, such as rheumatoid arthritis (Landini et al., 2022). Another study (Amin & Hosseinzadeh, 2012) examined behavioral changes caused by unilateral sciatic nerve ligation, including ipsilateral mechanical and thermal allodynia, with lesser thermal hyperalgesia. The researchers found that saffron extracts and safranal significantly reduced these symptoms over a week in a dose-related manner, with safranal being effective against thermal allodynia at high doses. However, crocin showed no improvement. Gabapentin, the reference drug, notably eased neuropathic pain symptoms. The authors proposed multiple mechanisms for saffron's effects, such as gamma-aminobutyric acid (GABA) receptor modulation, antioxidant activity, and extracellular glutamate reduction (Amin & Hosseinzadeh, 2012). One study (Tamaddonfard et al., 2014) explored safranal and vitamin E's impact on sciatic nerve injury recovery. They assessed functional recovery, neuropathic pain, histopathological changes, and blood MDA levels. Their findings suggested that ten days of safranal and vitamin E treatment hastened functional index values, eased cold and mechanical allodynia, improved Wallerian degeneration and muscle atrophy severity, and reversed MDA level increase (Tamaddonfard et al., 2014). Neuropathic pain involves heightened neuronal responses and the activation of non-neuronal cells, such as astrocytes and microglia, post-nerve damage. Activated cells release neuromodulators, including proinflammatory cytokines, perpetuating neuropathic pain (Kawasaki et al., 2008; Moalem & Tracey, 2006). One study explored safranal's effect on glial activation and cytokine production in a rat neuropathic pain model. Safranal effectively lessened pain sensitivity and curbed glial activation markers and proinflammatory cytokine (TNF-α and IL-1β) expression (Zhu & Yang, 2014). Table 1 represents the information from in vivo studies of the effects of saffron constituents on pain.
10 | ANTINOCICEPTIVE AND ANALGESIC ACTIVITIES OF SAFFRON ACCORDING TO CLINICAL STUDIES
Primary dysmenorrhea, painful menstrual cramps without identifiable pathological lesions, is a widespread gynecological concern affecting women, their families, and health services (Barcikowska et al., 2020; Dawood, 1981). Therapeutic approaches for dysmenorrhea, mainly focusing on suppressing prostaglandin synthesis, typically involve nonsteroidal antiinflammatories (NSAIDs) or oral contraceptives to lessen uterine contractions. However, the side effects of these medications prompt many to seek alternative treatments (Proctor & Farquhar, 2006). In a 2009 randomized, double-masked, placebo-controlled pilot trial, three groups of 18 to 27-year-old women with primary dysmenorrhea were given an herbal drug, mefenamic acid, or a placebo. The herbal treatment comprised a saffron, celery seed, and anise (SCA) extract. SCA users reported significant reductions in pain scores and durations, outperforming the other groups, with the study attributing these effects to SCA's antispasmodic and antiprostaglandin activity (Nahid et al., 2009).
After childbirth, women commonly experience uterine cramping or involution-related pain. Known as after-pain, it can be uncomfortable and may persist chronically in some cases (Deussen et al., 2011; Vermelis et al., 2010). In a single-blinded trial involving 108 postpartum women, the analgesic effects of a combination drug called PAC (Pimpinella anisum, Apium graveolens, and C. sativus) were compared to Mefenamic acid for after-pain relief. PAC and Mefenamic acid significantly reduced pain severity, but PAC exhibited greater efficacy and faster onset of pain relief. Only one PAC user reported dizziness, and no allergic symptoms were reported (Simbar et al., 2015).
One study (Mohammadierad et al., 2018) examined saffron impact on pain and anxiety in first-time mothers during labor. The study included 96 participants in Tabriz, Iran. They were divided into three groups: saffron plus date (Phoenix dactylifera L.) juice, saffron plus white sugar, and a placebo. The syrup was given orally every two hours, up to three doses. Results indicated lower pain and anxiety in the intervention groups, particularly the saffron plus date juice group. The saffron plus white sugar group did not show significant differences. It concluded that saffron plus date juice syrup can reduce pain and anxiety during labor (Mohammadierad et al., 2018). Fibromyalgia, a chronic disorder affecting 1%–10% of the population, primarily women, is characterized by widespread pain and various accompanying symptoms (Schmidt-Wilcke & Clauw, 2011). It carries a burden comparable to diabetes and hypertension (Ghavidel-Parsa et al., 2015). Major depressive disorder is highly prevalent among fibromyalgia patients (Gracely et al., 2012; Pae et al., 2008). Both fibromyalgia and major depressive disorder show decreased antioxidant capacity and increased oxidative stress (Cordero et al., 2010; Meeus et al., 2013). A clinical trial compared saffron and duloxetine for treating fibromyalgia symptoms. The treatments lasted eight weeks, and the two groups had no significant outcome differences (Shakiba et al., 2018). Muscular discomfort and pain concern athletes as they can hinder their training. Activities like downhill running, hopping, and weightlifting can lead to delayed-onset muscle soreness (DOMS), characterized by stiffness, tenderness, and pain (Connolly et al., 2003; Udani et al., 2009). DOMS is associated with muscle inflammation, damage, reduced strength, and limited joint range (Tartibian et al., 2009). The research studied saffron's effects on DOMS indicators in young male university students. The randomized, double-masked, placebo-controlled trial involved three groups: saffron, indomethacin, and control. Saffron and indomethacin both significantly prevented DOMS, with saffron being more effective. The saffron group showed increased isometric force post-exercise, while the control group had a decrease. The study highlights saffron's potential in mitigating DOMS and improving muscle performance. The saffron group also significantly reduced plasma lactate dehydrogenase (LDH) concentrations after 24, 48, and 72 h (Meamarbashi & Rajabi, 2014). In a randomized, double-blind, placebo-controlled clinical trial, Hamidi et al. (2020) investigated the effects of saffron supplementation on clinical outcomes, inflammatory markers, and oxidative stress in 63 female patients with active rheumatoid arthritis. The study found that saffron supplementation for 12 weeks significantly reduced rheumatoid arthritis disease activity, the number of tender and swollen joints, pain intensity, and erythrocyte sedimentation rate levels. However, the study did not find significant changes in levels of TNF-α, interferon-gamma (IFN-γ), high-sensitivity C-reactive protein (hs-CRP), MDA, and total antioxidant capacity (TAC) between the saffron and placebo groups. Also, no adverse effects were reported in the participants (Hamidi et al., 2020). Bozorgi et al. (2021) conducted a clinical trial to examine the effects of crocin on chemotherapy-induced peripheral neuropathy. A total of 177 patients received either a crocin tablet or a placebo twice daily for eight weeks. Crocin gradually improved chemotherapy-induced peripheral neuropathy scores compared to baseline, with no significant differences in adverse effects between the groups (Bozorgi et al., 2021). Table 2 represents the findings from clinical studies on the effects of saffron constituents.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Abbreviations: AIA, adjuvant-induced arthritis; BDNF, brain-derived neurotrophic factor; CCI, chronic constriction injury; COX, cyclooxygenase; CGRP, calcitonin gene-related peptide; ERK, extracellular signal-regulated kinase; IL-6, interleukin-6; iNOS, inducible NOS; JNKs, c-Jun N-terminal kinases; MDA, malondialdehyde; Nrf2, nuclear factor erythroid 2-elated factor 2; SCI, spinal cord injuries; SNI, spared nerve injury; SNT, spinal nerve transection; TRPA1, transient receptor potential ankyrin 1.
11 | TOXICITY OF SAFFRON AND ITS CONSTITUENTS IN ANIMAL STUDIES
The initial step in determining the toxicity of a chemical substance typically involves acute tests. These tests aim to establish the LD50 value, which is the dose that causes death in 50% of the test subjects, as well as other toxic effects, such as the impact on specific organs. This is usually done after administering the substance through one or more routes. Rats and mice are commonly used for these tests. The animals are observed for 14 days after receiving a single dose to assess lethality and other toxic effects (Bostan et al., 2017). The toxicity data on saffron safety are inconsistent. Studies have shown mice's LD50 values of saffron stigma and petal extracts were 1.6 and 6 g/kg, respectively, after IP exposure. The LD50 value of saffron was 4120 ± 556 mg/kg after oral administration in BALB/c mice (Hosseinzadeh et al., 2013). As for crocin, a significant constituent of saffron, it was observed that oral administration of 3 g/kg crocin over two days in mice did not result in death. A similar outcome was noted after IP exposure at the same dose.
Furthermore, IP administration of crocin at doses of 0.5, 1, 1.5, 2, and 3 g/kg did not cause death after 24 and 48 h. This suggests that crocin has a relatively low level of toxicity (Hosseinzadeh et al., 2010). The acute toxicity of safranal was studied, revealing LD50 values of 1.48, 1.88, and 1.50 mL/kg in male mice, female mice, and Wistar rats after IP administration. Oral administration altered these values to 21.42, 11.42, and 5.53 mL/kg, respectively. The maximum nonlethal dose in mice was 0.75 mL/kg, with significant differences in LD50 values between IP and oral administration because of first-pass metabolism and lower absorption (Hosseinzadeh et al., 2013). Subacute toxicity tests on rats revealed that saffron's ethanolic extract, administered IP at doses of 0.35–1.05 g/kg for two weeks, caused dose-dependent body weight decrease, hematological changes, and liver/ kidney injuries. The aqueous extract of saffron's petals and stigma also decreased body weight and caused hematological changes and organ injuries in rats (Karimi et al., 2004; Khayatnouri et al., 2011; Mohajeri et al., 2007).
Another study showed that oral administration of 200 mg/kg saffron for 28 days decreased the spermatogenesis index in rats. In contrast, a sub-acute study of rats using crocin (180 mg/kg) showed increased platelet/creatinine levels, decreased weight/food intake, and minor myosin light chain atrophy. Lower doses of crocin (90 mg/kg) resulted in altered albumin, alkaline phosphatase, and LDL levels, with no significant organ damage observed (Hosseinzadeh et al., 2010). A separate study examined the liver toxicity of crocin, finding that IP administration of 50, 100, and 200 mg/kg of crocin once a week for four weeks in rats did not alter serum parameters or cause significant toxicity (Taheri et al., 2014). The sub-acute toxicity of safranal was also studied, revealing that oral administration of safranal significantly affected hematological and biochemical parameters but did not cause noticeable lesions in different tissues, although it induced histopathological changes in the lung and kidney (Hosseinzadeh et al., 2013; Rezaee & Hosseinzadeh, 2013). In subchronic studies, the effects of saffron were examined in BALB/c mice over five weeks. High doses of saffron (4000 and 5000 mg/kg) led to decreased red and white blood cell counts and hemoglobin levels, increased BUN and creatinine levels indicative of kidney dysfunction, and raised liver enzyme activity. However, lower doses of saffron extract showed protective effects in different models (Muosa et al., 2015).
12 | DOSING, SAFETY, AND SIDE EFFECTS OF SAFFRON IN CLINICAL STUDIES
Saffron is considered safe for most people when consumed in the amounts typically found in food. In medicinal amounts, saffron has been safely used for six weeks. A specific combination product containing 30 mg of saffron has been used safely twice daily for up to 6 weeks. However, the dose of saffron may depend on several factors, such as the user's age, health, and other conditions (Akhondzadeh et al., 2004, 2005). The safety of saffron tablets (200 and 400 mg) was evaluated in healthy volunteers over seven days, revealing a decrease in mean arterial pressures and standing systolic blood pressure in those receiving the 400 mg dose. No significant hematological toxicity was observed (Ayatollahi et al., 2014; Modaghegh et al., 2008). Similarly, the safety of crocin tablets (20 mg) was assessed in a randomized, double-masked, placebo-controlled trial over one month, with results showing a decrease in partial thromboplastin time, amylase, and mixed WBC (Mohamadpour et al., 2013). A study on the impact of saffron exposure on miscarriage rates found a higher abortion rate among pregnant women exposed to high levels
TA BL E 2 Clinical studies of saffron and its constituents' effects on pain
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Abbreviations: CK, creatine kinase; LDH, lactate dehydrogenase; PAC, P. anisum, A. graveolens, and C. sativus; RA, XXX; VAS, XXX.
Of saffron (Ajam et al., 2014). In clinical studies, the administration of a saffron capsule (250 mg) at the beginning of active labor significantly reduced anxiety and fatigue scores and pain intensity during the first stage of labor, with no observed toxicity in infants and mothers (Ahmadi et al., 2015). Some side effects of saffron include dry mouth, anxiety, dizziness, drowsiness, nausea, change in appetite, and headache. Allergic reactions can occur in some people. Taking large amounts of saffron by mouth is possibly unsafe. High doses can cause poisoning, including the yellow appearance of the skin, eyes, and mucous membranes; vomiting; dizziness; bloody diarrhea; bleeding from the nose, lips, and eyelids; numbness; and other serious side effects (Lu et al., 2021). In conclusion, while saffron has many potential health benefits, it should be used cautiously because of possible side effects and interactions. Further research is needed to understand saffron's dosing, side effects, and interactions with other substances.
13 | INTERACTIONS OF SAFFRON WITH DIFFERENT MEDICATIONS AND DRUGS
One study found that saffron was as effective as fluoxetine, a selective serotonin reuptake inhibitor (SSRI), in treating mild-to-moderate depression. This suggests that saffron might interact with SSRIs and other antidepressants, possibly enhancing their effects (Kashani et al., 2013). CNS depressants, also known as sedatives and tranquilizers, are substances that can slow brain activity. They are often used to treat anxiety and sleep disorders. When combined with other substances that have similar effects, such as saffron, the result can be an enhanced effect, leading to potentially dangerous levels of sedation. Several studies suggest that saffron and its constituents, crocin and safranal, have various pharmacological, soothing, and hypnotic effects. This might enhance the effects of CNS depressants, leading to increased sedation or drowsiness (Jackson et al., 2021; Siddiqui et al., 2018). In a meta-analysis of randomized clinical trials of saffron and major depressive disorders, it was shown that saffron may have antidepressant properties, which might interact with CNS depressants. The study found that saffron supplementation can improve symptoms in patients with major depressive disorder (Hausenblas et al., 2013).
Read about the Antidepressant Properties Of Crocin Molecules In Saffron
14 | CONCLUSION
This narrative review provides a comprehensive overview of the analgesic properties of saffron and its constituents, including crocin, safranal, and Picrocrocin. The paper meticulously synthesizes a wealth of preclinical and clinical research demonstrating the potential of saffron as a natural analgesic in various pain conditions, including neuropathic pain, inflammatory pain, and cancer-induced pain.
The review's key findings highlight the multi-modal action of saffron and its constituents in pain modulation. This includes inhibiting inflammatory pathways, reducing oxidative stress, modulating neurotransmitter systems, and interacting with opioid receptors. These findings are significant as they suggest that saffron might be used as a natural alternative or adjunct to conventional analgesics, which often have undesirable side effects.
However, while the review provides a robust synthesis of the current literature, several limitations exist. First, various saffron extracts were used in several studies, but a clear rationale for the chosen doses was not mentioned. This makes it difficult to determine the optimal dosage for achieving analgesic effects.
Second, some studies did not provide a detailed analysis of the chemical constituents of the saffron extract used. This information would be valuable for understanding the potential active ingredients contributing to the observed effects.
Third, several studies did not explore the potential long-term effects, side effects, toxicity, or adverse reactions of saffron and its constituents alone or in combination with other drugs. This is a significant limitation, as the safety profile of a potential analgesic is just as crucial as its efficacy.
Fourthly, several studies did not explore the exact mechanism through which saffron and its constituents exert their analgesic effects. Understanding these mechanisms is crucial for optimizing using saffron as an analgesic and predicting its impact on different pain conditions.
Fifthly, several studies relied on a single animal model of pain, which may limit the generalizability of their findings to other types of pain. Finally, several studies had relatively small sample sizes, which could restrict their statistical power and the reliability of their findings.
The review also underscores the need for future research in this area. Given the promising findings from preclinical studies, there is a clear need for more rigorous clinical trials to validate these results in human populations. These should ideally be randomized controlled trials with larger sample sizes, which would provide more robust evidence of the efficacy of saffron as an analgesic. Future research should also explore saffron's optimal dosage and administration route and its constituents for pain management.
From a clinical practice perspective, this review has significant implications for health professionals. Given the increasing prevalence of chronic pain conditions and the limitations of current analgesics, there is a pressing need for alternative or adjunctive treatments. The findings of this review suggest that saffron might fill this gap. However, health professionals should exercise caution and ensure that using saffron for pain management is based on robust clinical evidence tailored to the patient's needs and circumstances.
Regarding policy development and implementation, this review highlights the potential of natural products like saffron in pain management. Policymakers should consider supporting research and developing guidelines for using natural products in pain management. This could include regulations to ensure the quality and safety of these products, as well as education and training for health professionals on their use.
Please send your full-text request to our email address info[at]flonita.com
Note: in your mail, please put the paper title in the subject or mail body section or use the share link button provided at the end of that post to share your look-up page with us.
Original paper reference; Pourbagher‐Shahri AM, Forouzanfar F. Saffron (Crocus sativus) and its constituents for pain management: A review of current evidence. Phytotherapy Research. 2023 Nov;37(11):5041-57.
Ahmadi, S., Azhari, S., Jafarzadeh, H., Rakhshandeh, H., & Mazlom, R. (2015). The effect of oral capsules of saffron on anxiety and fatigue during the first stage of labor. Journal of Shahid Sadoughi University of Medical Sciences, 23(2), 1915–1926.
Ait Tastift, M., Makbal, R., Bourhim, T., Omari, Z., Isoda, H., & Gadhi, C. (2022). Safety assessment and pain relief properties of saffron from Taliouine region (Morocco). Molecules (Basel, Switzerland), 27(10), 3339.
Ajam, M., Reyhani, T., Roshanravan, V., & Zare, Z. (2014). Increased miscarriage rate in female farmers working in saffron fields: A possible effect of saffron toxicity. Asia Pacific Journal of Medical Toxicology, 3(2), 73–75.
Akhondzadeh, S., Fallah-Pour, H., Afkham, K., Jamshidi, A. H., & KhalighiCigaroudi, F. (2004). Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: A pilot double-blind randomized trial [ISRCTN45683816]. BMC Complementary and Alternative Medicine, 4, 12.
Akhondzadeh, S., Tahmacebi-Pour, N., Noorbala, A. A., Amini, H., FallahPour, H., Jamshidi, A. H., & Khani, M. (2005). Crocus sativus L. in the treatment of mild to moderate depression: A double-blind, randomized and placebo-controlled trial. Phytotherapy Research: PTR, 19(2), 148–151.
Amin, B., Abnous, K., Motamedshariaty, V., & Hosseinzadeh, H. (2014). Attenuation of oxidative stress, inflammation and apoptosis by ethanolic and aqueous extracts of Crocus sativus L. stigma after chronic constriction injury of rats. Anais da Academia Brasileira de Ciências, 86(4), 1821–1832.
Amin, B., Hosseini, S., & Hosseinzadeh, H. (2017). Enhancement of antinociceptive effect by co-administration of amitriptyline and Crocus sativus in a rat model of neuropathic pain. Iranian Journal of
Pharmaceutical Research: IJPR, 16(1), 187–200.
Amin, B., & Hosseinzadeh, H. (2012). Evaluation of aqueous and ethanolic extracts of saffron, Crocus sativus L., and its constituents, safranal and crocin in allodynia and hyperalgesia induced by chronic constriction injury model of neuropathic pain in rats. Fitoterapia, 83(5), 888–895.
Ansari, M. Y., Ahmad, N., & Haqqi, T. M. (2020). Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomedicine & Pharmacotherapy, 129, 110452.
Argoff, C. (2011). Mechanisms of pain transmission and pharmacologic management. Current Medical Research and Opinion, 27(10), 2019– 2031.
Asdaq, S. M. B., & Inamdar, M. N. (2010). Potential of Crocus sativus (saffron) and its constituent, crocin, as hypolipidemic and antioxidant in rats. Applied Biochemistry and Biotechnology, 162(2), 358–372.
Asgharzade, S., Talaei, A., Farkhondeh, T., & Forouzanfar, F. (2020). A review on stem cell therapy for neuropathic pain. Current Stem Cell Research & Therapy, 15(4), 349–361.
Attal, N., Lanteri-Minet, M., Laurent, B., Fermanian, J., & Bouhassira, D. (2011). The specific disease burden of neuropathic pain: Results of a French nationwide survey. Pain, 152(12), 2836–2843.
Ayatollahi, H., Javan, A. O., Khajedaluee, M., Shahroodian, M., & Hosseinzadeh, H. (2014). Effect of Crocus sativus L. (saffron) on coagulation and anticoagulation systems in healthy volunteers. Phytotherapy Research: PTR, 28(4), 539–543.
Azami, S., Shahriari, Z., Asgharzade, S., Farkhondeh, T., Sadeghi, M., Ahmadi, F., Vahedi, M. M., & Forouzanfar, F. (2021). Therapeutic potential of saffron (Crocus sativus L.) in ischemia stroke. Evidencebased Complementary and Alternative Medicine, 2021, 1–8.
Barcikowska, Z., Rajkowska-Labon, E., Grzybowska, M. E., HansdorferKorzon, R., & Zorena, K. (2020). Inflammatory markers in dysmenorrhea and therapeutic options. International Journal of Environmental Research and Public Health, 17(4), 1191.
Basbaum, A. I., Bautista, D. M., Scherrer, G., & Julius, D. (2009). Cellular and molecular mechanisms of pain. Cell, 139(2), 267–284.
Besson, J. M. (1999). The neurobiology of pain. Lancet (London, England), 353(9164), 1610–1615.
Bingham, B., Ajit, S. K., Blake, D. R., & Samad, T. A. (2009). The molecular basis of pain and its clinical implications in rheumatology. Nature Clinical Practice. Rheumatology, 5(1), 28–37.
Bostan, H. B., Mehri, S., & Hosseinzadeh, H. (2017). Toxicology effects of saffron and its constituents: A review. Iranian Journal of Basic Medical Sciences, 20(2), 110–121.
Bozorgi, H., Ghahremanfard, F., Motaghi, E., Zamaemifard, M., Zamani, M., & Izadi, A. (2021). Effectiveness of crocin of saffron (Crocus sativus L.) against chemotherapy-induced peripheral neuropathy: A randomized, double-blind, placebo-controlled clinical trial. Journal of Ethnopharmacology, 281, 114511.
Caballero-Ortega, H., Pereda-Miranda, R., Riveron-Negrete, L., Hernández, J. M., Medécigo-Ríos, M., Castillo-Villanueva, A., & Abdullaev, F. I. (2004). Chemical composition of saffron (Crocus sativus L.) from four countries. Acta Horticulturae, 650, 321–326.
Campbell, J. N., & Meyer, R. A. (2006). Mechanisms of neuropathic pain. Neuron, 52(1), 77–92.
Chen, W., Walwyn, W., Ennes, H. S., Kim, H., McRoberts, J. A., & Marvizon, J. C. (2014). BDNF released during neuropathic pain poten- tiates NMDA receptors in primary afferent terminals. European Journal of Neuroscience, 39(9), 1439–1454.
Choy, E. H. (2015). The role of sleep in pain and fibromyalgia. Nature Reviews Rheumatology, 11(9), 513–520.
Chrysanthou, A., Pouliou, E., Kyriakoudi, A., & Tsimidou, M. Z. (2016). Sensory threshold studies of picrocrocin, the major bitter compound of saffron. Journal of Food Science, 81(1), S189–S198.
Cid-Pérez, T. S., Nevárez-Moorillon, G. V., Ochoa-Velasco, C. E., Navarro-Cruz, A. R., Hernández-Carranza, P., & Avila-Sosa, R. (2021). The relation between drying conditions and the development of volatile compounds in saffron (Crocus sativus). Molecules (Basel, Switzerland), 26(22), 6954.
Connolly, D. A., Sayers, S. P., & McHugh, M. P. (2003). Treatment and prevention of delayed onset muscle soreness. Journal of Strength and Conditioning Research, 17(1), 197–208.
Cordero, M. D., de Miguel, M., Carmona-Lopez, I., Bonal, P., Campa, F., & Moreno-Fernández, A. M. (2010). Oxidative stress and mitochondrial dysfunction in fibromyalgia. Neuro Endocrinology Letters, 31(2), 169–173.
Courtney, C. A., Fernández-de-Las-Peñas, C., & Bond, S. (2017). Mechanisms of chronic pain – Key considerations for appropriate physical therapy management. The Journal of Manual & Manipulative Therapy, 25(3), 118–127.
Dawood, M. Y. (1981). Dysmenorrhoea and prostaglandins: Pharmacological and therapeutic considerations. Drugs, 22(1), 42–56.
de Oliveira, D. L., Hirotsu, C., Tufik, S., & Andersen, M. L. (2017). The interfaces between vitamin D, sleep and pain. The Journal of Endocrinology, 234(1), R23–r36.
Deussen, A. R., Ashwood, P., & Martis, R. (2011). Analgesia for relief of pain due to uterine cramping/involution after birth. Cochrane Database of Systematic Reviews, 5, CD004908.
Erfanparast, A., Tamaddonfard, E., & Henareh-Chareh, F. (2020). Central H2 histaminergic and alpha-2 adrenergic receptors involvement in crocetin-induced antinociception in orofacial formalin pain in rats. Veterinary Research Forum, 11(3), 229–234.
Erfanparast, A., Tamaddonfard, E., Taati, M., & Dabbaghi, M. (2015). Effects of crocin and safranal, saffron constituents, on the formalin-induced orofacial pain in rats. Avicenna Journal of Phytomedicine, 5(5), 392–402.
Farshid, A., & Tamaddonfard, E. (2015). Histopathological and behavioral evaluations of the effects of crocin, safranal and insulin on diabetic peripheral neuropathy in rats. Avicenna Journal of Phytomedicine, 5(5), 469–478.
Fernández-Albarral, J. A., Ramírez, A. I., de Hoz, R., Lopez-Villarín, N., Salobrar-García, E., Lopez-Cuenca, I., Licastro, E., InarejosGarcía, A. M., Almodovar, P., Pinazo-Durán, M. D., Ramírez, J. M., & Salazar, J. J. (2019). Neuroprotective and anti-inflammatory effects of a hydrophilic saffron extract in a model of glaucoma. International Journal of Molecular Sciences, 20(17), 4110.
Finnerup, N. B., Kuner, R., & Jensen, T. S. (2021). Neuropathic pain: From mechanisms to treatment. Physiological Reviews, 101(1), 259–301.
Fishman, S. M. (2007). Recognizing pain management as a human right: A first step. LWW. Forouzanfar, F. (2022). Interplay between heat shock proteins, inflammation and pain: A promising therapeutic approach. Current Molecular Pharmacology, 15(1), 170–178.
Forouzanfar, F., Hosseinzadeh, H., Khorrami, M. B., Asgharzade, S., & Rakhshandeh, H. (2019). Attenuating effect of Portulaca oleracea extract on chronic constriction injury-induced neuropathic pain in rats: An evidence of anti-oxidative and anti-inflammatory effects. CNS & Neurological Disorders Drug Targets, 18(4), 342–349.
Forouzanfar, F., Pourbagher-Shahri, A. M., & Ghazavi, H. (2022). Evaluation of antiarthritic and antinociceptive effects of cedrol in a rat model of arthritis. Oxidative Medicine and Cellular Longevity, 2022, 1–10.
Forouzanfar, F., Tanha, N. K., Pourbagher-Shahri, A. M., Mahdianpour, S., Esmaeili, M., & Ghazavi, H. (2023). Synergistic effect of ellagic acid and gabapentin in a rat model of neuropathic pain. Metabolic Brain Disease, 38(4), 1421–1432.
Ghavidel-Parsa, B., Bidari, A., Amir Maafi, A., & Ghalebaghi, B. (2015). The iceberg nature of fibromyalgia burden: The clinical and economic aspects. The Korean Journal of Pain, 28(3), 169–176.
Goldberg, D. S., & McGee, S. J. (2011). Pain as a global public health priority. BMC Public Health, 11(1), 1–5.
Gracely, R. H., Ceko, M., & Bushnell, M. C. (2012). Fibromyalgia and depression. Pain Research and Treatment, 2012, 486590.
Guedes, R. P., Bosco, L. D., Teixeira, C. M., Araújo, A. S., Llesuy, S., Bello- Klein, A., Ribeiro, M. F. M., & Partata, W. A. (2006). Neuropathic pain modifies antioxidant activity in rat spinal cord. Neurochemical Research, 31(5), 603–609.
Guindon, J., & Hohmann, A. G. (2009). The endocannabinoid system and pain. CNS & Neurological Disorders Drug Targets, 8(6), 403–421.
Guo, Z.-L., Li, M.-X., Li, X.-L., Wang, P., Wang, W.-G., Du, W.-Z., Yang, Z.-Q., Chen, S.-F., & Di Wu, X.-Y. T. (2022). Crocetin: A systematic review. Frontiers in Pharmacology, 12, 3920.
Hamidi, Z., Aryaeian, N., Abolghasemi, J., Shirani, F., Hadidi, M., Fallah, S., & Moradi, N. (2020). The effect of saffron supplement on clinical outcomes and metabolic profiles in patients with active rheumatoid arthritis: A randomized, double-blind, placebo-controlled clinical trial. Phytotherapy Research: PTR, 34(7), 1650–1658.
Hausenblas, H. A., Saha, D., Dubyak, P. J., & Anton, S. D. (2013). Saffron (Crocus sativus L.) and major depressive disorder: A meta-analysis of randomized clinical trials. Journal of Integrative Medicine, 11(6), 377–383.
Hicks, C. W., & Selvin, E. (2019). Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Current Diabetes Reports, 19(10), 86.
Hosseini, A., Razavi, B. M., & Hosseinzadeh, H. (2018). Pharmacokinetic properties of saffron and its active components. European Journal of Drug Metabolism and Pharmacokinetics, 43(4), 383–390.
Hosseinzadeh, H., Sadeghi Shakib, S., Khadem Sameni, A., & Taghiabadi, E. (2013). Acute and subacute toxicity of safranal, a constituent of saffron, in mice and rats. Iranian Journal of Pharmaceutical Research: IJPR, 12(1), 93–99.
Hosseinzadeh, H., Shariaty, V. M., & Sameni, A. K. (2010). Acute and subacute toxicity of crocin, aconstituent of Crocus sativus L. (saffron), in mice and rats. Pharmacology, 2, 943–951.
Hosseinzadeh, H., & Shariaty, V. M. J. P. (2007). Anti-nociceptive effect of safranal, a constituent of Crocus sativus (saffron), in mice. Pharmacologyonline, 2, 498–503.
Hosseinzadeh, H., & Younesi, H. M. (2002). Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacology, 2, 7.
Hunskaar, S., & Hole, K. (1987). The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain, 30(1), 103–114.
Ilie, M. A., Caruntu, C., Tampa, M., Georgescu, S.-R., Matei, C., Negrei, C., Ion, R. M., Constantin, C., Neagu, M., & Boda, D. (2019). Capsaicin: Physicochemical properties, cutaneous reactions and potential applications in painful and inflammatory conditions. Experimental and Therapeutic Medicine, 18(2), 916–925.
Izzo, A. A., Hoon-Kim, S., Radhakrishnan, R., & Williamson, E. M. (2016). A critical approach to evaluating clinical efficacy, adverse events and drug interactions of herbal remedies. Phytotherapy Research, 30(5), 691–700.
Jackson, P. A., Forster, J., Khan, J., Pouchieu, C., Dubreuil, S., Gaudout, D., Moras, B., Pourtau, L., Joffre, F., Vaysse, C., Bertrand, K., Abrous, H., Vauzour, D., Brossaud, J., Corcuff, J. B., Capuron, L., & Kennedy, D. O. (2021). Effects of saffron extract supplementation on mood, well-being, and response to a psychosocial stressor in healthy adults: A randomized, double-blind, parallel group, clinical trial. Frontiers in Nutrition, 7, 606124.
José Bagur, M., Alonso Salinas, G. L., Jiménez-Monreal, A. M., Chaouqi, S., Llorens, S., Martínez-Tomé, M., & Alonso, G. (2017). Saffron: An old medicinal plant and a potential novel functional food. Molecules (Basel, Switzerland), 23(1), 30.
Kadoglou, N. P., Christodoulou, E., Kostomitsopoulos, N., & Valsami, G. (2021). The cardiovascular-protective properties of saffron and its potential pharmaceutical applications: A critical appraisal of the literature. Phytotherapy Research, 35(12), 6735–6753.
Karami, M., Bathaie, S. Z., Tiraihi, T., Habibi-Rezaei, M., Arabkheradmand, J., & Faghihzadeh, S. (2013). Crocin improved locomotor function and mechanical behavior in the rat model of contused spinal cord injury through decreasing calcitonin gene related peptide (CGRP). Phytomedicine, 21(1), 62–67.
Karimi, G., Taiebi, N., Hosseinzadeh, H., & Shirzad, F. (2004). Evaluation of subacute toxicity of aqueous extract of Crocus sativus L. stigma and petal in rats. Journal of Medicinal Plants, 3(12), 29–35.
Kashani, L., Raisi, F., Saroukhani, S., Sohrabi, H., Modabbernia, A., Nasehi, A. A., Jamshidi, A., Ashrafi, M., Mansouri, P., Ghaeli, P., & Akhondzadeh, S. (2013). Saffron for treatment of fluoxetine-induced sexual dysfunction in women: Randomized double-blind placebocontrolled study. Human Psychopharmacology, 28(1), 54–60.
Kawasaki, Y., Zhang, L., Cheng, J. K., & Ji, R. R. (2008). Cytokine mechanisms of central sensitization: Distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. The Journal of Neuroscience, 28(20), 5189–5194.
Khayatnouri, M., Safavi, S. E., Safarmashaei, S., Babazadeh, D., & Mikailpourardabili, B. J. A. E. B. (2011). The effect of saffron orally administration on spermatogenesis index in rat. Advances in Environmental Biology, 5(7), 1514–1521.
Khorasanchi, Z., Shafiee, M., Kermanshahi, F., Khazaei, M., Ryzhikov, M., Parizadeh, M. R., Kermanshahi, B., Ferns, G. A., Avan, A., & Hassanian, S. M. (2018). Crocus sativus a natural food coloring and flavoring has potent anti-tumor properties. Phytomedicine, 43, 21–27.
Kobayashi, K., Fukuoka, T., Obata, K., Yamanaka, H., Dai, Y., Tokunaga, A., & Noguchi, K. (2005). Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. The Journal of Comparative Neurology, 493(4), 596–606.
Landini, L., Monteiro, S., de Araujo, D., Titiz, M., Geppetti, P., Nassini, R., & De Logu, F. (2022). TRPA1 role in inflammatory disorders: What is known so far? International Journal of Molecular Sciences, 23(9), 4529.
Lei, M., Guo, C., Hua, L., Xue, S., Yu, D., Zhang, C., & Wang, D. (2017). Crocin attenuates joint pain and muscle dysfunction in osteoarthritis rats. Inflammation, 40(6), 2086–2093.
Li Puma, S., Landini, L., Macedo, S. J., Jr., Seravalli, V., Marone, I. M., Coppi, E., Patacchini, R., Geppetti, P., Materazzi, S., Nassini, R., & de Logu, F. (2019). TRPA1 mediates the antinociceptive properties of the constituent of Crocus sativus L., safranal. Journal of Cellular and Molecular Medicine, 23(3), 1976–1986.
Loeser, R. F., Goldring, S. R., Scanzello, C. R., & Goldring, M. B. (2012). Osteoarthritis: A disease of the joint as an organ. Arthritis and Rheumatism, 64(6), 1697–1707.
Lories, R. J., Corr, M., & Lane, N. E. (2013). To Wnt or not to Wnt: The bone and joint health dilemma. Nature Reviews Rheumatology, 9(6), 328–339.
Lu, C., Ke, L., Li, J., Zhao, H., Lu, T., Mentis, A. F. A., Wang, Y., Wang, Z., Polissiou, M. G., Tang, L., Tang, H., & Yang, K. (2021). Saffron (Crocus sativus L.) and health outcomes: A meta-research review of metaanalyses and an evidence mapping study. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology., 91, 153699.
Maggi, M. A., Bisti, S., & Picco, C. (2020). Saffron: Chemical composition and neuroprotective activity. Molecules (Basel, Switzerland), 25(23), 5618.
Maghsoodi, V., Kazemi, A., & Akhondi, E. (2012). Effect of different drying methods on saffron (Crocus sativus L) quality. Iranian Journal of Chemistry & Chemical Engineering, 31(2), 85–89.
Maqbool, Z., Arshad, M. S., Ali, A., Aziz, A., Khalid, W., Afzal, M. F., Bangar, S. P., Addi, M., Hano, C., & Lorenzo, J. M. (2022). Potential role of phytochemical extract from saffron in development of functional foods and protection of brain-related disorders. Oxidative Medicine and Cellular Longevity, 2022, 6480590.
Markenson, J. A. (1996). Mechanisms of chronic pain. The American Journal of Medicine, 101(1a), 6s–18s.
Mashmoul, M., Azlan, A., Khaza'ai, H., Mohd Yusof, B. N., & Mohd Noor, S. (2013). Saffron: A natural potent antioxidant as a promising anti-obesity drug. Antioxidants, 2(4), 293–308.
Matsuzawa-Yanagida, K., Narita, M., Nakajima, M., Kuzumaki, N., Niikura, K., Nozaki, H., Takagi, T., Tamai, E., Hareyama, N., Terada, M., Yamazaki, M., & Suzuki, T. (2008). The usefulness of antidepressants for improving the neuropathic pain-like state and pain-induced anxiety through actions at different brain sites. Neuropsychopharmacology, 33(8), 1952–1965.
McNamara, C. R., Mandel-Brehm, J., Bautista, D. M., Siemens, J., Deranian, K. L., Zhao, M., Hayward, N. J., Chong, J. A., Julius, D., Moran, M. M., & Fanger, C. M. (2007). TRPA1 mediates formalin-induced pain. Proceedings of the National Academy of Sciences of the United States of America, 104(33), 13525–13530.
Meamarbashi, A., & Rajabi, A. (2014). Preventive effects of 10-day supplementation with saffron and indomethacin on delayed-onset muscle soreness. Clinical Journal of Sport Medicine, 25, 25–112.
Meeus, M., Nijs, J., Hermans, L., Goubert, D., & Calders, P. (2013). The role of mitochondrial dysfunctions due to oxidative and nitrosative stress in chronic pain or chronic fatigue syndromes and fibromyalgia patients: Peripheral and central mechanisms as therapeutic targets? Expert Opinion on Therapeutic Targets, 17(9), 1081–1089.
Mercer Lindsay, N., Chen, C., Gilam, G., Mackey, S., & Scherrer, G. (2021). Brain circuits for pain and its treatment. Science Translational Medicine, 13(619), eabj7360.
Moalem, G., & Tracey, D. J. (2006). Immune and inflammatory mechanisms in neuropathic pain. Brain Research Reviews, 51(2), 240–264.
Modaghegh, M. H., Shahabian, M., Esmaeili, H. A., Rajbai, O., & Hosseinzadeh, H. (2008). Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytomedicine, 15(12), 1032–1037.
Mohajeri, D., Mousavi, G., Mesgari, M., Doustar, Y., & Khayat Nouri, M. (2007). Subacute toxicity of Crocus sativus L.(saffron) stigma ethanolic extract in rats. American Journal of Pharmacology and Toxicology, 2(4), 189–193.
Mohamadpour, A. H., Ayati, Z., Parizadeh, M. R., Rajbai, O., & Hosseinzadeh, H. (2013). Safety evaluation of crocin (a constituent of saffron) tablets in healthy volunteers. Iranian Journal of Basic Medical Sciences, 16(1), 39–46.
Mohammadierad, R., Mohammad-Alizadeh-Charandabi, S., Mirghafourvand, M., & Fazil, F. (2018). Effect of saffron with or without date sugar on intensity of pain and anxiety during labor in primiparous females: A randomized, controlled trial. Iranian Red Crescent Medical Journal, 20(1), 1–8.
Muley, M. M., Krustev, E., & McDougall, J. J. (2016). Preclinical assessment of inflammatory pain. CNS Neuroscience & Therapeutics, 22(2), 88–101.
Muosa, F., Al-Rekabi, K., Askar, S., & Yousif, E. (2015). Evaluation of the toxic effect of ethanolic extract of saffron in male mice after subchronic exposure. Donnish Journal of Pharmacy and Pharmacology, 1, 1–7.
Musazadeh, V., Zarezadeh, M., Faghfouri, A. H., Keramati, M., Ghoreishi, Z., & Farnam, A. (2022). Saffron, as an adjunct therapy, contributes to relieve depression symptoms: An umbrella meta-analysis. Pharmacological Research, 175, 105963.
Nahid, K., Fariborz, M., Ataolah, G., & Solokian, S. (2009). The effect of an Iranian herbal drug on primary dysmenorrhea: A clinical controlled trial. Journal of Midwifery & Women's Health, 54(5), 401–404.
Nassini, R., Materazzi, S., Benemei, S., & Geppetti, P. (2014). The TRPA1 channel in inflammatory and neuropathic pain and migraine. Reviews of Physiology, Biochemistry and Pharmacology, 167, 1–43.
Negah, S. S., Ghazavi, H., Vafaee, F., Rashidi, R., Aminian, A. R., & Forouzanfar, F. (2021). The potential role of green tea and its main constituent (epigallocatechin-3-gallate) in pain relief: A mechanistic review. Current Drug Discovery Technologies, 18(6), e130921189586.
Negah, S. S., Hajinejad, M., Nemati, S., Roudbary, S. M. J. M., & Forouzanfar, F. (2023). Stem cell therapy combined with luteolin alleviates experimental neuropathy. Metabolic Brain Disease, 38(6), 1895– 1903.
Nestler, E. J. (2004). Molecular mechanisms of drug addiction. Neuropharmacology, 47(Suppl. 1), 24–32.
Nicholson, B., & Verma, S. (2004). Comorbidities in chronic neuropathic pain. Pain Medicine (Malden, Mass), 5(Suppl. 1), S9–s27.
Nilius, B., & Szallasi, A. (2014). Transient receptor potential channels as drug targets: From the science of basic research to the art of medicine. Pharmacological Reviews, 66(3), 676–814.
Ossipov, M. H., Morimura, K., & Porreca, F. (2014). Descending pain modulation and chronification of pain. Current Opinion in Supportive and Palliative Care, 8(2), 143–151.
Pae, C. U., Luyten, P., Marks, D. M., Han, C., Park, S. H., Patkar, A. A., Masand, P. S., & van Houdenhove, B. (2008). The relationship between fibromyalgia and major depressive disorder: A comprehensive review.
Current Medical Research and Opinion, 24(8), 2359–2371.
Patel, S., Sarwat, M., & Khan, T. H. (2017). The mechanism behind the antitumor potential of saffron (Crocus sativus L.): The molecular perspective. Critical Reviews in Oncology/Hematology, 115, 27–35.
Pitsikas, N. (2016). Constituents of saffron (Crocus sativus L.) as potential candidates for the treatment of anxiety disorders and schizophrenia. Molecules (Basel, Switzerland), 21(3), 303.
Posadas, I., Bucci, M., Roviezzo, F., Rossi, A., Parente, L., Sautebin, L., & Cirino, G. (2004). Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. British Journal of Pharmacology, 142(2), 331–338.
Predieri, S., Magli, M., Gatti, E., Camilli, F., Vignolini, P., & Romani, A. (2021). Chemical composition and sensory evaluation of saffron. Foods (Basel, Switzerland), 10(11), 2604.
Proctor, M., & Farquhar, C. (2006). Diagnosis and management of dysmenorrhoea. BMJ, 332(7550), 1134–1138.
Raboisson, P., & Dallel, R. (2004). The orofacial formalin test. Neuroscience and Biobehavioral Reviews, 28(2), 219–226.
Raffaeli, W., & Arnaudo, E. (2017). Pain as a disease: An overview. Journal of Pain Research, 10, 2003–2008.
Rahaiee, S., Hashemi, M., Moini, S., Shojaosadati, S. A., & Razavi, S. (2015). Comparison of phytochemical constituents and antioxidant activities of aqueous and alcoholic extracts of saffron. Quality Assurance & Safety of Crops and Food, 7(4), 521–529.
Rakhshandeh, H., Ghorbanzadeh, A., Negah, S. S., Akaberi, M., Rashidi, R., & Forouzanfar, F. (2021). Pain-relieving effects of Lawsonia inermis on neuropathic pain induced by chronic constriction injury. Metabolic Brain Disease, 36, 1709–1716.
Rezaee, R., & Hosseinzadeh, H. (2013). Safranal: From an aromatic natural product to a rewarding pharmacological agent. Iranian Journal of Basic Medical Sciences, 16(1), 12–26.
Rezaei, F., Saebipour, M. R., Ghaemi, K., Hassanzadeh-Taheri, M. M., Foadoddini, M., & Hosseini, M. (2020). Intra-cerebroventricular administration of crocin attenuates sleep deprivation-induced hyperalgesia in rats. Basic and Clinical Neuroscience, 11(3), 261–267.
Roehrs, T., Hyde, M., Blaisdell, B., Greenwald, M., & Roth, T. (2006). Sleep loss and REM sleep loss are hyperalgesic. Sleep, 29(2), 145–151.
Rogério, F., Teixeira, S. A., de Rezende, A. C., de Sá, R. C., de Souza, Q. L., De Nucci, G., Muscará, M. N., & Langone, F. (2005). Superoxide dismutase isoforms 1 and 2 in lumbar spinal cord of neonatal rats after sciatic nerve transection and melatonin treatment. Brain Research. Developmental Brain Research, 154(2), 217–225.
Rosenfeld, J., Cook, S., & James, R. (1997). Expression of superoxide dismutase following axotomy. Experimental Neurology, 147(1), 37–47.
Rubio Moraga, A., Ahrazem, O., Rambla, J. L., Granell, A., & Gomez Gomez, L. (2013). Crocins with high levels of sugar conjugation con- tribute to the yellow colours of early-spring flowering crocus tepals.
PLoS One, 8(9), e71946.
Safakhah, H. A., Damghanian, F., Bandegi, A. R., & Miladi-Gorji, H. (2020). Effect of crocin on morphine tolerance and serum BDNF levels in a rat model of neuropathic pain. Pharmacological Reports: PR, 72(2), 305–313.
Safakhah, H. A., Taghavi, T., Rashidy-Pour, A., Vafaei, A. A., Sokhanvar, M., Mohebbi, N., & Rezaei-Tavirani, M. (2016). Effects of saffron (Crocus sativus L.) stigma extract and its active constituent crocin on neuropathic pain responses in a rat model of chronic constriction injury. Iranian Journal of Pharmaceutical Research: IJPR, 15(1), 253–261.
Schmidt-Wilcke, T., & Clauw, D. J. (2011). Fibromyalgia: From pathophysiology to therapy. Nature Reviews Rheumatology, 7(9), 518–527.
Schou, W. S., Ashina, S., Amin, F. M., Goadsby, P. J., & Ashina, M. (2017). Calcitonin gene-related peptide and pain: A systematic review. The Journal of Headache and Pain, 18(1), 34.
Schutte, I. W., Akkermans, L. M., & Kroese, A. B. (1997). CCKA and CCKB receptor subtypes both mediate the effects of CCK-8 on myenteric neurons in the Guinea-pig ileum. Journal of the Autonomic Nervous System, 67(1–2), 51–59.
Setty, A. R., & Sigal, L. H. (Eds.). (2005). Herbal medications commonly used in the practice of rheumatology: Mechanisms of action, efficacy, and side effects. Seminars in arthritis and rheumatism. Elsevier.
Shahi, T., Assadpour, E., & Jafari, S. M. (2016). Main chemical compounds and pharmacological activities of stigmas and tepals of ‘red gold’; saffron. Trends in Food Science and Technology, 58, 69–78.
Shakiba, M., Moazen-Zadeh, E., Noorbala, A. A., Jafarinia, M., Divsalar, P., Kashani, L., Shahmansouri, N., Tafakhori, A., Bayat, H., & Akhondzadeh, S. (2018). Saffron (Crocus sativus) versus duloxetine for treatment of patients with fibromyalgia: A randomized, double-blind clinical trial. Avicenna Journal of Phytomedicine, 8(6), 513–523.
Siddiqui, M. J., Saleh, M. S. M., Basharuddin, S., Zamri, S. H. B., Mohd Najib, M. H. B., Che Ibrahim, M. Z. B., Binti Mohd Noor, N. A., Binti Mazha, H. N., Hassan, N. M., & Khatib, A. (2018). Saffron (Crocus sativus L.): As an antidepressant. Journal of Pharmacy & Bioallied Sciences, 10(4), 173–180.
Simbar, M., Shadipour, M., Salamzadeh, J., Ramezani-Tehrani, F., & Nasiri, N. (2015). The combination of “Pimpinella anisum, Apium graveolens, and Crocus sativus (PAC)” is more effective than “mefenamic acid” on postpartum after-pain. Journal of Herbal Medicine, 5(1), 20–25.
Taheri, F., Bathaie, S. Z., Ashrafi, M., & Ghasemi, E. (2014). Assessment of crocin toxicity on the rat liver. Pathobiology Research, 17(3), 67–79.
Tamaddonfard, E., Farshid, A. A., Eghdami, K., Samadi, F., & Erfanparast, A. (2013). Comparison of the effects of crocin, safranal and diclofenac on local inflammation and inflammatory pain responses induced by carrageenan in rats. Pharmacological Reports: PR, 65(5), 1272–1280.
Tamaddonfard, E., Farshid, A. A., Maroufi, S., Kazemi-Shojaei, S., Erfanparast, A., Asri-Rezaei, S., Taati, M., Dabbaghi, M., & Escort, M. (2014). Effects of safranal, a constituent of saffron, and vitamin E on nerve functions and histopathology following crush injury of sciatic nerve in rats. Phytomedicine, 21(5), 717–723.
Tamaddonfard, E., & Hamzeh-Gooshchi, N. (2010a). Effect of crocin on the morphine-induced antinociception in the formalin test in rats. Phytotherapy Research: PTR, 24(3), 410–413.
Tamaddonfard, E., & Hamzeh-Gooshchi, N. (2010b). Effects of intraperitoneal and intracerebroventricular injection of crocin on acute corneal pain in rats. Phytotherapy Research: PTR, 24(10), 1463–1467.
Tamaddonfard, E., Tamaddonfard, S., & Pourbaba, S. (2015). Effects of intra-fourth ventricle injection of crocin on capsaicin-induced orofacial pain in rats. Avicenna Journal of Phytomedicine, 5(5), 450.
Tartibian, B., Maleki, B. H., & Abbasi, A. (2009). The effects of ingestion of omega-3 fatty acids on perceived pain and external symptoms of delayed onset muscle soreness in untrained men. Clinical Journal of Sport Medicine, 19(2), 115–119.
Tjølsen, A., Berge, O. G., Hunskaar, S., Rosland, J. H., & Hole, K. (1992). The formalin test: An evaluation of the method. Pain, 51(1), 5–17.
Trujillo, K. A., & Akil, H. (1991). Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science (New York, N.Y.), 251(4989), 85–87.
Udani, J. K., Singh, B. B., Singh, V. J., & Sandoval, E. (2009). BounceBack capsules for reduction of DOMS after eccentric exercise: A randomized, double-blind, placebo-controlled, crossover pilot study. Journal of the International Society of Sports Nutrition, 6, 14.
Vafaei, A. A., Safakhah, H. A., Jafari, S., Tavasoli, A., Rashidy-Pour, A., Ghanbari, A., Seyedinia, S. A., & Tarahomi, P. (2020). Role of cannabinoid receptors in crocin-induced hypoalgesia in neuropathic pain in rats. Journal of Experimental Pharmacology, 12, 97–106.
Venkatachalam, K., & Montell, C. (2007). TRP channels. Annual Review of Biochemistry, 76, 387–417.
Vermelis, J. M., Wassen, M. M., Fiddelers, A. A., Nijhuis, J. G., & Marcus, M. A. (2010). Prevalence and predictors of chronic pain after labor and delivery. Current Opinion in Anaesthesiology, 23(3), 295–299.
Wang, J.-F., Xu, H.-J., He, Z.-L., Yin, Q., & Cheng, W. (2020). Crocin alleviates pain hyperalgesia in AIA rats by inhibiting the spinal Wnt5a/βcatenin signaling pathway and glial activation. Neural Plasticity, 2020, 4297483.
Wang, X., Zhang, G., Qiao, Y., Feng, C., & Zhao, X. (2017). Crocetin attenuates spared nerve injury-induced neuropathic pain in mice. Journal of Pharmacological Sciences, 135(4), 141–147.
Wang, Y., Chen, S., Du, K., Liang, C., Wang, S., Boadi, E. O., Li, J., Pang, X., He, J., & Chang, Y.-X. (2021). Traditional herbal medicine: Therapeutic potential in rheumatoid arthritis. Journal of Ethnopharmacology, 279, 114368.
Williams, J. T., Ingram, S. L., Henderson, G., Chavkin, C., von Zastrow, M., Schulz, S., Koch, T., Evans, C. J., & Christie, M. D. J. (2013). Regulation of μ-opioid receptors: Desensitization, phosphorylation, internalization, and tolerance. Pharmacological Reviews, 65(1), 223–254.
Winterhalter, P., & Straubinger, M. (2000). Saffron—Renewed interest in an ancient spice. Food Reviews International, 16(1), 39–59.
Woolf, C. J. (2004). Pain: Moving from symptom control toward mechanism-specific pharmacologic management. Annals of Internal Medicine, 140(6), 441–451.
Yaribeygi, H., Zare, V., Butler, A. E., Barreto, G. E., & Sahebkar, A. (2019). Antidiabetic potential of saffron and its active constituents. Journal of Cellular Physiology, 234(6), 8610–8617.
Yu, W.-H. A. (2002). Spatial and temporal correlation of nitric oxide synthase expression with CuZn-superoxide dismutase reduction in motor neurons following axotomy. Annals of the New York Academy of Sciences, 962(1), 111–121.
Zhao, H., Alam, A., Chen, Q., Eusman, M. A., Pal, A., Eguchi, S., Wu, L., & Ma, D. (2017). The role of microglia in the pathobiology of neuropathic pain development: What do we know? British Journal of Anaesthesia, 118(4), 504–516.
Zhu, K.-J., & Yang, J.-S. (2014). Anti-allodynia effect of safranal on neuropathic pain induced by spinal nerve transection in rat. International Journal of Clinical and Experimental Medicine, 7(12), 4990–4996.
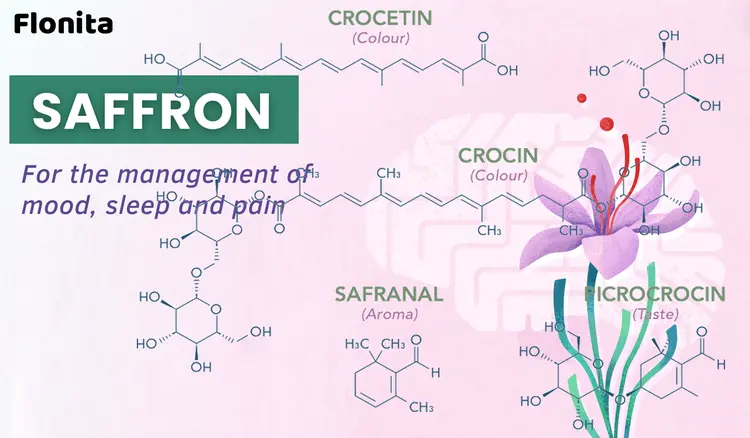
Your Comment
Required fields*